PROFESSIONAL INFORMATION FOR HUMAN MEDICINES
SCHEDULING STATUS
| S1 |
1 NAME OF THE MEDICINE
iliadin® Aloe
Strength
Each 1 ml solution contains oxymetazoline hydrochloride 0,5 mg/0,05 % m/v
Pharmaceutical form
Nasal spray, solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each 1 ml solution contains:
0,5 mg/0,05 % m/v Oxymetazoline hydrochloride
Benzalkonium chloride 0,02 % m/v and Benzyl alcohol 0,2 % m/v as preservative
Contains sweetener: Acesulfame potassium 0,15 mg & Sorbitol 71,4 mg per 1 ml
Contains sodium: 2,066 mg per 1 ml
For a full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Nasal spray, solution
The product is a clear liquid with a colourless to very slight yellowish colouration, free from particulate and suspended matter with a characteristic aromatic odour resembling aroma of eucalyptus and menthol.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
iliadin Aloe is indicated for the relief of nasal congestion due to the common cold (acute rhinitis), sinusitis, allergic rhinitis.
Indicated as adjunctive treatment in middle ear infection.
4.2 Posology and method of administration
Posology
Adults: 1-2 sprays into each nostril 2 – 3 times a day.
Paediatric population
Children over 6 years: 1 spray into each nostril 2 - 3 times a day.
Not intended for children below the age of 6 years.
The single dose given for iliadin Aloe must not be administered more than 3 times a day. Do not administer for longer than 5 days. Do not exceed the recommended dosage. If symptoms persist, consult a health care provider.
Method of Administration
Metered Nose Spray for nasal use.
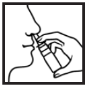
For best results keep both the head and spray bottle in an upright position. Remove cap by pulling and insert the nozzle of the spray loosely into the nostril with the middle and forefinger around the bottom of the nozzle and the thumb on the base of the bottle. Depress the spray mechanism (as shown in the diagram) thus producing a fine mist. Sniff in to ensure an even distribution of the spray. Withdraw from nostril and release the spray mechanism. Repeat for the other nostril.
4.3 Contraindications
Hypersensitivity to the active ingredient, benzalkonium chloride or any of the other ingredients listed in section 6.1.
Rhinitis sicca (inflammation of the skin and mucosa of the nasal vestibule and encrustation).
Children below six years of age.
In patients following a trans-sphenoidal hypophysectomy.
4.4 Special warnings and precautions for use
In the following cases, iliadin Aloe may only be used after carefully weighing the risk-to-benefit ratio:
Patients treated with monoamine oxidase inhibitors or, have taken MAOIs in the previous two weeks or, tricyclic antidepressants, and other medicines such as stated in 4.5.
Medicines potentially increasing blood pressure.
Increased intraocular pressure, especially narrow-angle glaucoma.
Severe cardiovascular diseases (e.g. coronary heart disease, angina, hypertension, cardiac asthma).
Phaeochromocytoma.
Metabolic disorders (e.g. hyperthyroidism, diabetes mellitus, porphyria).
Hyperplasia of the prostate.
Do not exceed the recommended dose.
If symptoms worsen or do not improve after 3 days, physician should re-evaluate clinical situations.
iliadin Aloe should be used for a maximum of 5 consecutive days to avoid a rebound-effect and drug-induced rhinitis.
Long-term use and overdosage of iliadin Aloe should be avoided.
The efficacy iliadin Aloe may be reduced (tachyphylaxis) with long-term use or overdose. This may lead to use of higher doses or to more frequent usage which, in turn, can result in permanent use. If long term use or overdose occurs, treatment should be discontinued immediately.
Continuous use may cause nasal congestion due to reactive hyperaemia of the nasal mucosa (rebound effect) and chronic swelling of the nasal mucosa (rhinitis medicamentosa) as well as mucosal atrophy or rhinitis sicca. Rebound effects and tachyphylaxis should stop once use of iliadin Aloe is discontinued.
Medical supervision is indicated in patients with chronic rhinitis. Dosages higher than recommended may only be used under medical supervision.
4.5 Interaction with other medicines and other forms of interaction
The concomitant use of iliadin Aloe and certain mood-stimulating medicines with hypertensive effect (e.g. MAO inhibitors and tricyclic antidepressants) may lead to an increase in blood pressure or hypertensive crisis due to their cardiovascular activity.
The efficacy of beta-blocking drugs such as methyldopa, bethanidine, debrisoquine and guanethidine or other anti-hypertensive drugs may be reduced with concomitant use of iliadin Aloe.
Possible additive cardiovascular toxicity may occur when sympathomimetics are given with antiparkinsonian drugs such as bromocriptine.
4.6 Fertility, pregnancy and lactation
Pregnancy and lactation
iliadin Aloe should only be used after the consultation with a medical practitioner during pregnancy and lactation.
The recommended dosage must not be exceeded.
Fertility
No data are available on the effects of iliadin Aloe on human fertility.
4.7 Effects on ability to drive and use machines
No impairment is to be expected if used as recommended.
Systemic effects with involvement of the cardiovascular or central nervous system cannot be excluded after prolonged administration of iliadin Aloe or intake of oxymetazoline containing cold remedies in doses higher than recommended. In these cases, the ability to drive a vehicle or operate machinery can be impaired.
4.8 Undesirable effects
Immune system disorders:
Frequency unknown: Hypersensitivity reactions (angioedema, rash, pruritus).
Psychiatric disorders:
Frequency unknown: Insomnia, restlessness.
Nervous system disorders:
Less frequent: Headache or light-headedness, nervousness, anxiety, irritability.
Frequency unknown: Somnolence, sedation, hallucinations, convulsions.
Cardiac disorders:
Frequency unknown: Palpitations, tachycardia.
Vascular disorders:
Frequency unknown: Hypertension (increased blood pressure).
Respiratory, thoracic and mediastinal disorders:
Frequent: Aqueous nasal secretions.
Frequent: Crusted nose.
Less frequent: Rebound congestion (increase in runny or stuffy nose).
Frequency unknown: Nasal discomfort (burning of the nasal mucosa), nasal dryness, sneezing, after the effect has worn off increased swelling of the mucosa (reactive hyperaemia), epistaxis.
General disorders and administration site conditions:
Frequency unknown: Fatigue, tachyphylaxis (associated with long-term use or overdose). Systemic effects have occurred after local administration. If unexpected side effects appear, a medical practitioner should be consulted immediately.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicine is important. It allows continued monitoring of the benefit/risk balance of the medicine. Health care providers are asked to report any suspected adverse reactions to SAHPRA via the “6.04 Adverse Drug Reactions Reporting Form”, found online under SAHPRA’s publications: https://www.sahpra.org.za/Publications/Index/8
4.9 Overdose
Overdosage may occur after nasal or accidental oral administration.
The efficacy of iliadin Aloe may be reduced (tachyphylaxis) with long-term use or overdose. This may lead to use of higher doses or to more frequent usage which, in turn, can result in permanent use. If long term use or overdose occurs, treatment should be discontinued immediately.
The clinical picture following intoxication with imidazol-derivatives may be unclear due to the occurrence of episodes of hyperactivity alternated with episodes of depression of the central nervous system and of the cardiovascular and pulmonary system.
Symptoms
Symptoms of an overdose may be:
Hypertension, tachycardia, palpitations, cardiac dysrhythmia, cardiac arrest, sweating, agitation, convulsion, mydriasis, nausea, vomiting, cyanosis, fever, spasms, circulatory collapse, pulmonary oedema, respiratory disorders, psychic disorders, drowsiness, paleness, miosis, decrease in body temperature, bradycardia, shock-like hypotension, apnoea, loss of consciousness and coma.
In children above 6 years, in particular, overdose often causes dominating central nervous effects with convulsions and coma, bradycardia, apnoea as well as hypertension possibly followed by hypotension.
Therapeutic measures after overdosage
In-house intensive-care therapy is indicated in cases of severe overdose. Administration of medicinal charcoal (absorbent) or sodium sulfate (laxative) should be performed rapidly.
A non-selective alpha-blocker can be given as antidote. If required, initiate fever lowering measures, anticonvulsive therapy and oxygen ventilation.
Vasopressors are contraindicated.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Sympathomimetics, plain.
ATC code: R01AA05
Category and class: A.16.1 Nasal Decongestants
Oxymetazoline is a direct-acting sympathomimetic amine. It acts on alpha-adrenergic receptors in the arterioles of the nasal mucosa to produce constriction, resulting in decreased blood flow and decreased nasal congestion.
Application of oxymetazoline into the nostrils leads to decongestion of the inflamed nasal mucosa and thus to a normalization of nasal breathing.
5.2 Pharmacokinetic properties
Absorption
The effect of oxymetazoline sets in within a few minutes.
The effect of oxymetazoline persists for up to 12 hours.
Relevant absorption of pharmacodynamically effective doses of oxymetazoline following the recommended topical use is regarded as uncommon but cannot be excluded.
The absorption rate is estimated at 3,5 hours. The maximum plasma concentration can be found after 8 to 10 hours.
Elimination
Terminal serum half-life is 35 hours, and the excretion measured in faeces (1,1 % of the applied dose, after 48 hours) and urine (2,1 % of the applied dose, after 96 hours).
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Sorbitol
Sodium citrate dihydrate (for pH-adjustment)
Polysorbate 80
Benzyl alcohol
Citric acid, anhydrous (for pH-adjustment)
Benzalkonium chloride solution
Acesulfame potassium
Levomenthol
Cineole (eucalypthol)
Disodium edetate
Aloe dry extract
Levocarvone
Water, purified.
6.2 Incompatibilities
Not applicable
6.3 Shelf life
Glass bottle 15 ml: 3 years
Use within 12 months of first opening the bottle.
6.4 Special precautions for storage
Store at or below 25 °C.
Protect from light. Keep well closed.
6.5 Nature and contents of container
15 ml Brown glass bottle with a metering pump (polypropylene).
6.6 Special precautions for disposal
No special requirements.
7 HOLDER OF CERTIFICATE OF REGISTRATION
P&G South African Trading (Pty) Ltd. 10 th Floor, Alice Lane Towers, 15 Alice Lane Sandton, 2196 South Africa
8 REGISTRATION NUMBER(S)
50/16.1/0335
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
20 September 2022
